
Predicting Products Worksheet Answers Printable Word Searches
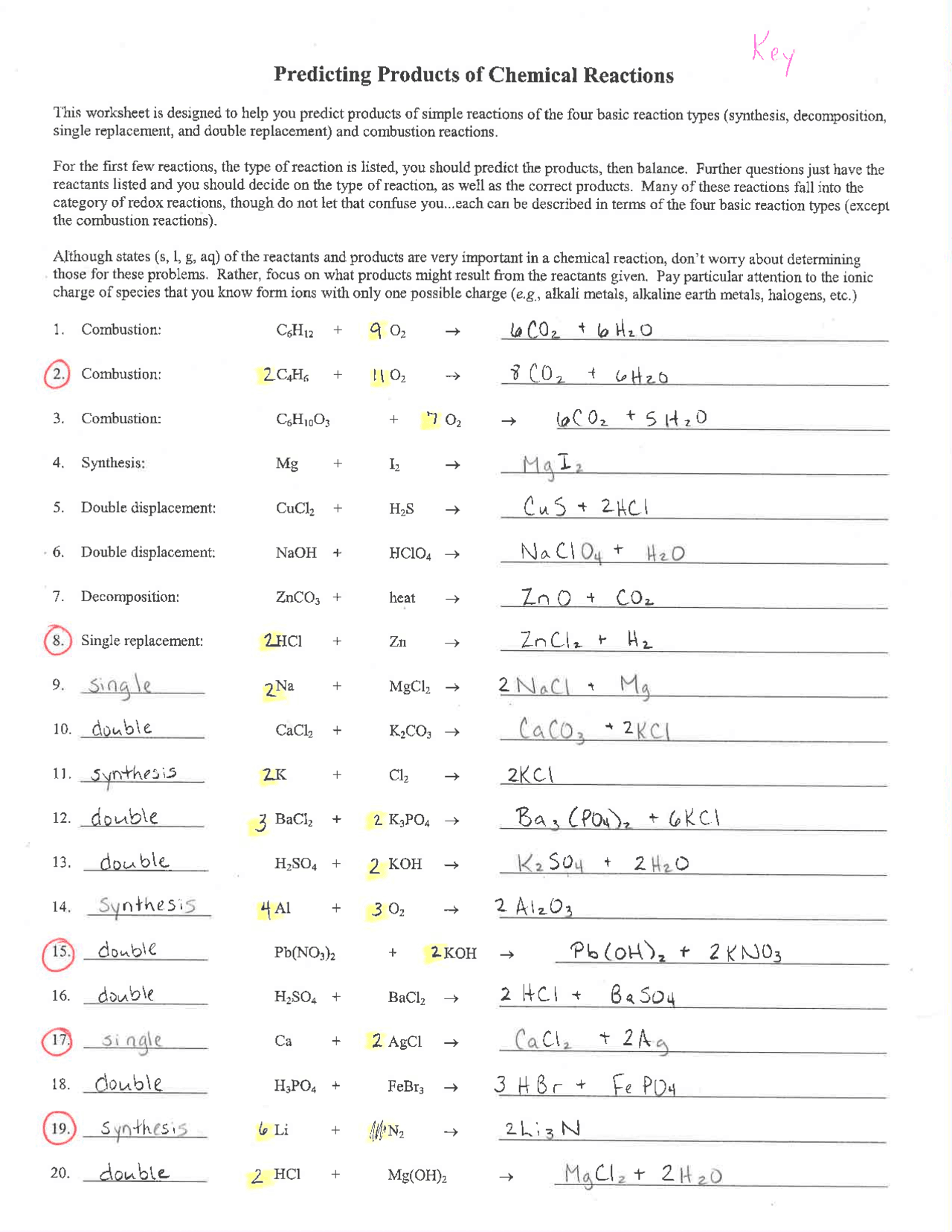
Predicting Products of Chemical Reactions This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and combustion reactions. For the first few reactions, the type of reaction is listed, you should predict the products, then.
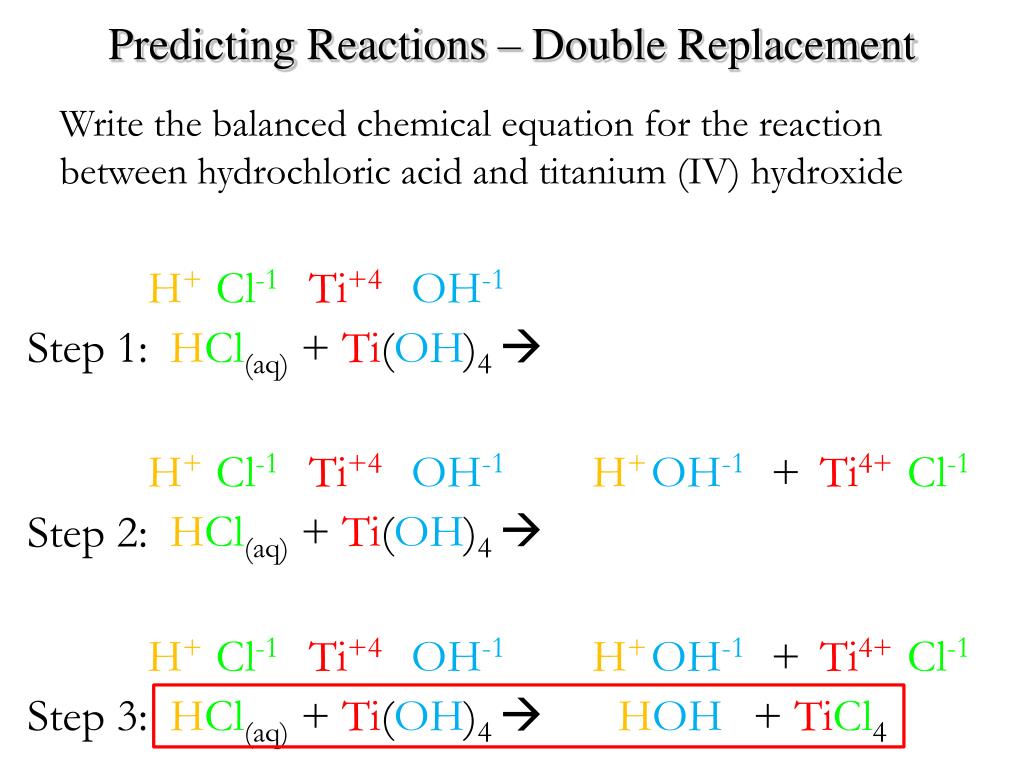
PPT Chemical Reactions Predicting Products and Balancing PowerPoint Presentation ID4272005
This worksheet provides students with 23 opportunities to predict the products of synthesis, combustion, decomposition, single replacement, and double replacement reactions. An answer key is provided. Can accompany Modern Chemistry by Holt, Rinehart, and Winston (1st edition) textbook. Check out my TPT store for accompanying notes, worksheets.

6 Predicting Products Of Chemical Reactions Worksheet FabTemplatez
Purpose: Predicting the products and balancing chemical reactions is the main skillset that students need to have leaving this chapter. I start teaching this with synthesis reactions, because they are one of the simplest and most straightforward forms of chemical reactions. This worksheet provides a few examples of how to predict products of.

Predicting Products of Chemical Reactions Foldable Math = Love
Predicting Products of Chemical Reactions A. Predict the products in the following reactions that have been labeled as synthesis (S), decomposition (D), single-replacement (SR) double-replacement (DR), and combustion (C). Balance the equations. l) 4) 5) 6) 8) c. DR. SR s: DR CHO cuC12 Na cu N02 cao + MgCl AgN03 + Mg(NO ) B. Predict the products.

Predicting products of chemical reactions
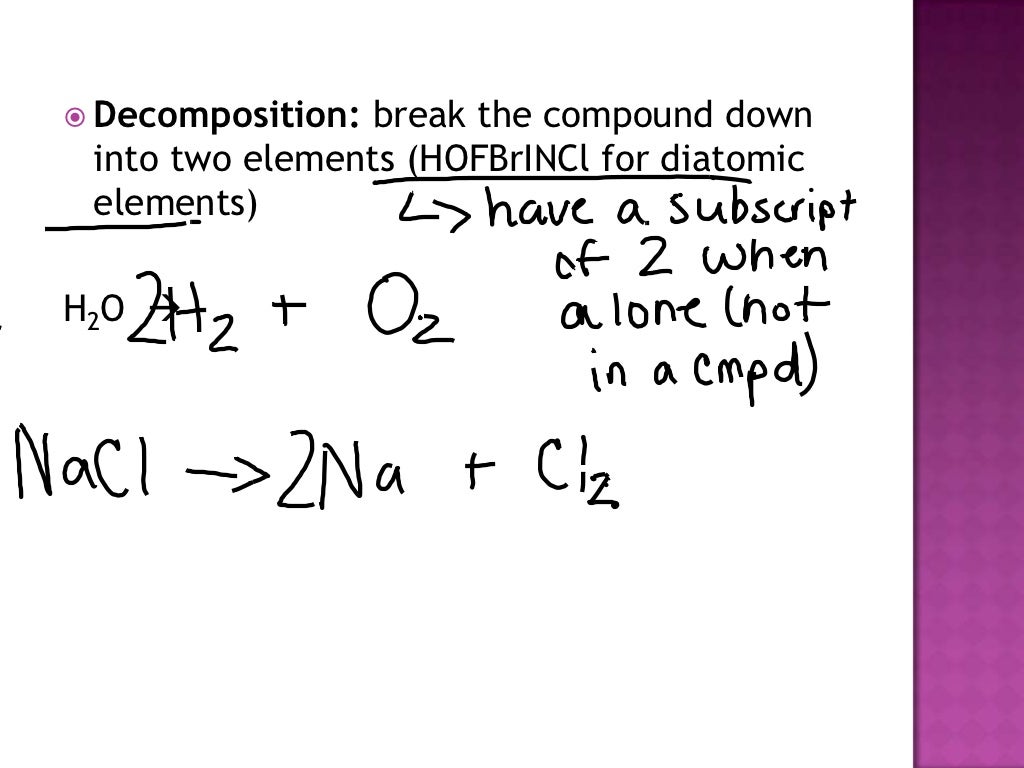
2 HgO (s) → O 2 (g) + 2 Hg (l) 2 KClO 3 (s) → 3 O 2 (g) + 2 KCl (s) The potential products in double-replacement reactions are simple to predict; the anions and cations simply exchange. Remember, however, that one of the products must precipitate, otherwise no chemical reaction has occurred. For the reaction between lead (II) nitrate and.
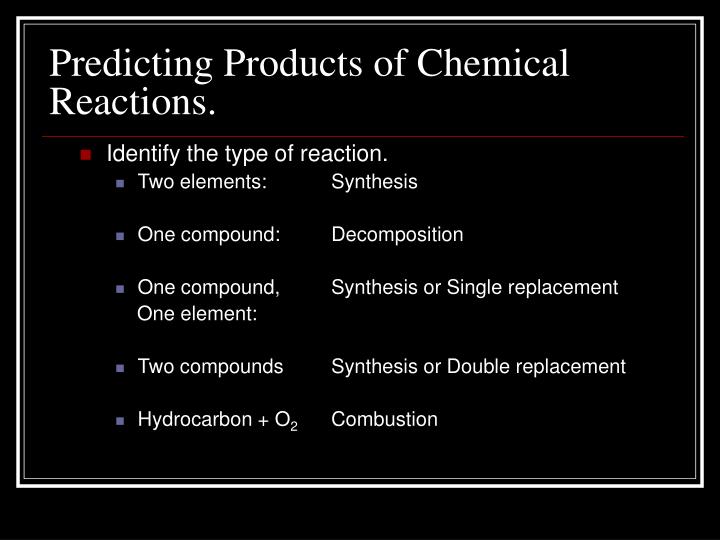
PPT Predicting Products of Chemical Reactions. PowerPoint Presentation ID3407988
Types of chemical reactions. Oxidation-reduction (redox) reactions. Worked example: Using oxidation numbers to identify oxidation and reduction. Balancing redox equations. Dissolution and precipitation. Precipitation reactions. Double replacement reactions. Single replacement reactions. Molecular, complete ionic, and net ionic equations.
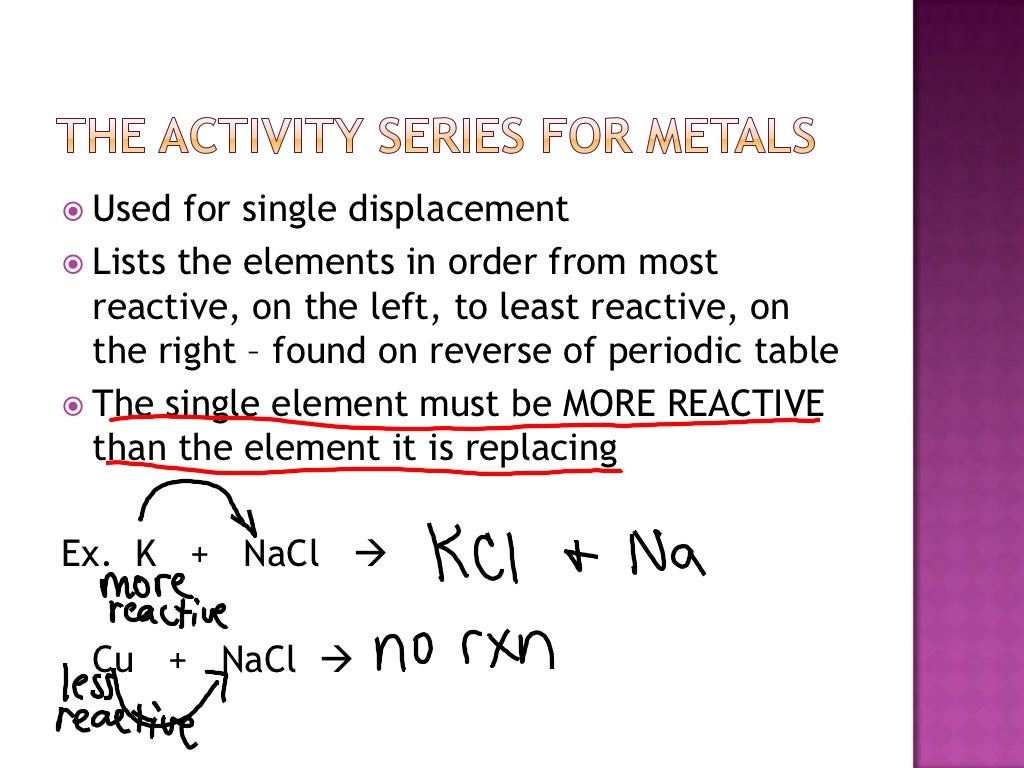
Predicting products of chemical reactions
*Reaction proceeds to completion. 2. Write the formula of the reactants. Predict the products that will form from the reactants given, write the chemical formulas and name them if possible. Balance the chemical equation. Name the type of chemical reaction. a) magnesium reacts with iodine gas b) aluminum oxide reacts with iron(II) chloride
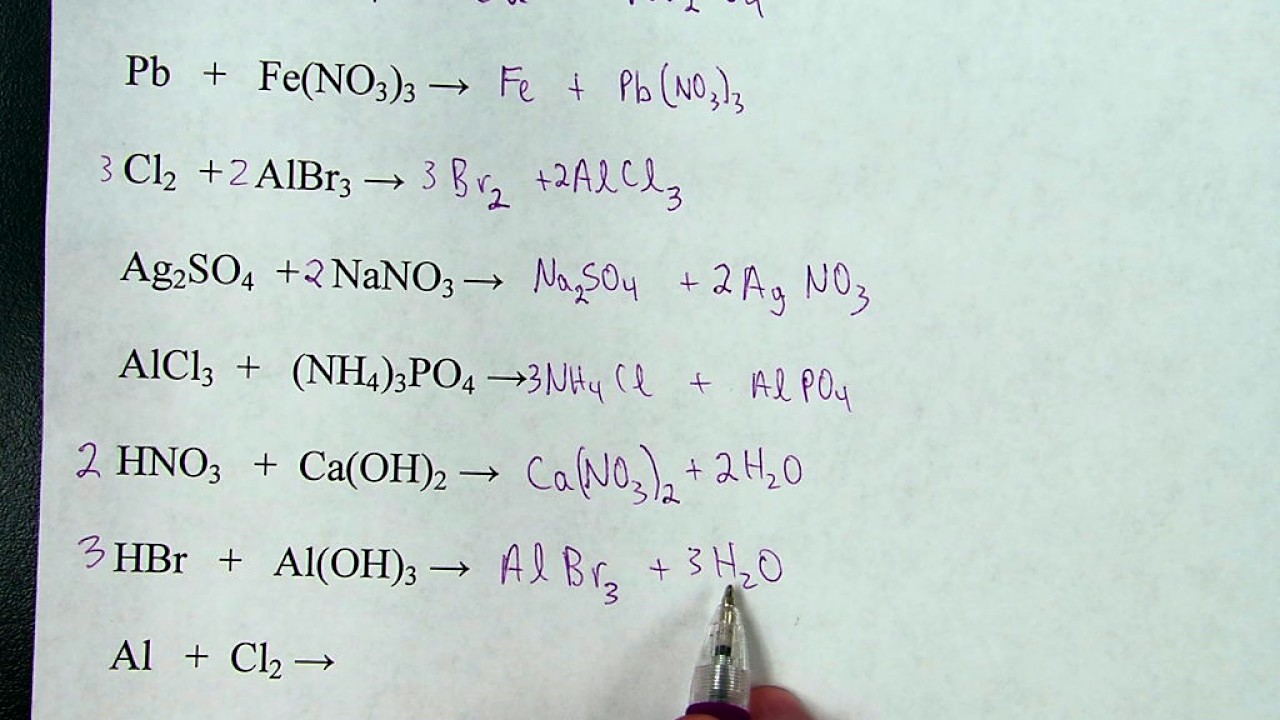
Predicting Products Of Chemical Reactions Practice Worksheet Answers Master of
Predicting Products Of Chemical Reactions Worksheets 2024. Chemists frequently need to make predictions about the chemical reactions that will occur when two substances are mixed. For instance, we would be disappointed if we were unable to foresee an explosive response when adding a chemical to a tank of dangerous waste in order to stabilize it.
Predicting products of chemical reactions
Predict the products of the reactions below. If a precipitate forms, indicate by circling the precipitate. 1. silver nitrate + zinc chloride → 2. zinc nitrate + potassium iodide → 3. Iron III chloride + sodium hydroxide →. Title: Predicting Products of Chemical Reactions Worksheet Author:
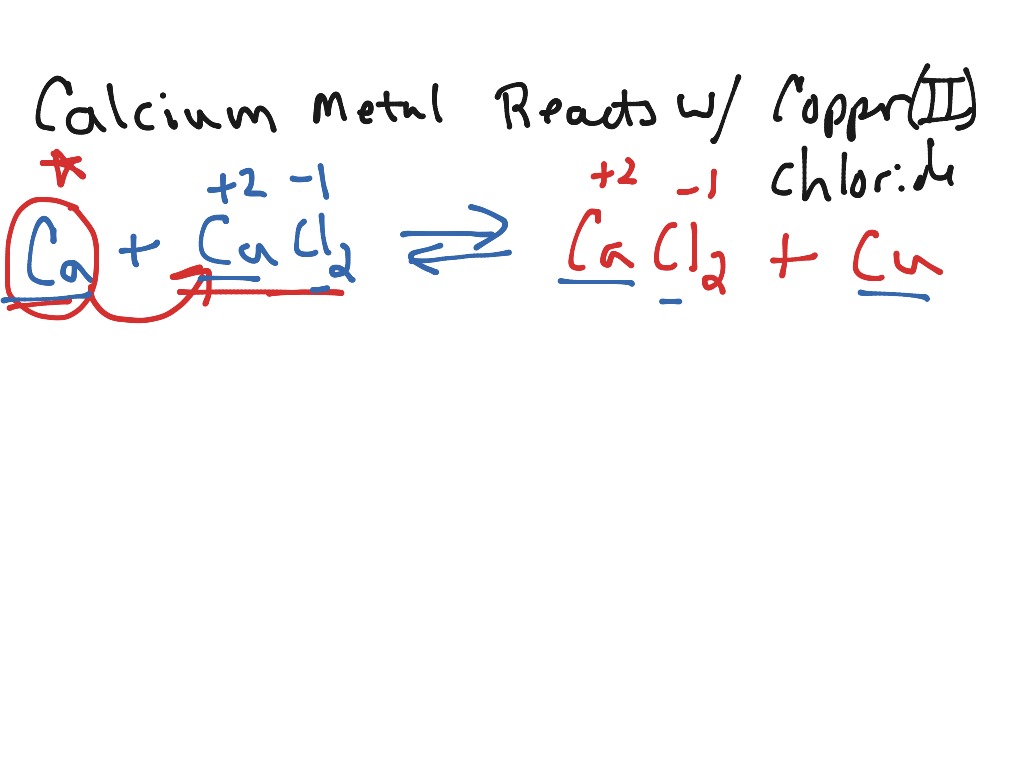
Predicting Products Science, Chemistry, Chemicalreactions ShowMe
Predicting Products of Chemical Reactions Worksheet. Predict the products of the reactions below. Then, write the balanced equation and classify the reaction. If a precipitate forms, indicate by using (s) by the precipitate. If "no reaction" occurs write N.R. beside the question. Use your activity series, predicting products helper sheet.

Predicting Reaction Products Worksheets Answers
Balancing chemical equations 1. Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O. Note: All reactants and products require a coefficient of at least one. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with.

Predicting Products Of Chemical Reactions Worksheet Answers —
Step 1. Predicting Products of Chemical Reactions Worksheet dict the products of the reactions below. Then, write the balanced equation and classify the reaction. If a precipitate forms, indicate by using (s) by the precipitate "no reaction" occurs write N.R. beside the question. Use your activity series, predicting products helper sheet and.
Predicting Chemical Reactions Worksheet
HCl + NaOH →NaCl + H₂O (acid base) C₆H₁₂ + O₂ →. C₆H₁₂ + 9O₂ → 6CO₂ + 6H₂O (combustion) Li + S →. 2Li + S → Li₂S (synthesis) Look at the reactants of a chemical reaction, identify the type of reaction, and predict the products. For this set you will have to balance the equations….

Predicting Products Worksheet Answer Key
CHM 130 Predicting Products Worksheet Circle the appropriate reaction type for each, complete the reaction with products (remember to check charges for ionic compounds), include states, and finally balance each reaction. CB = combustion, AB = acid base neutralization, SR = single replacement, DR = double replacement, NR = no reaction 1.
16 Types Chemical Reactions Worksheets Answers Free PDF at
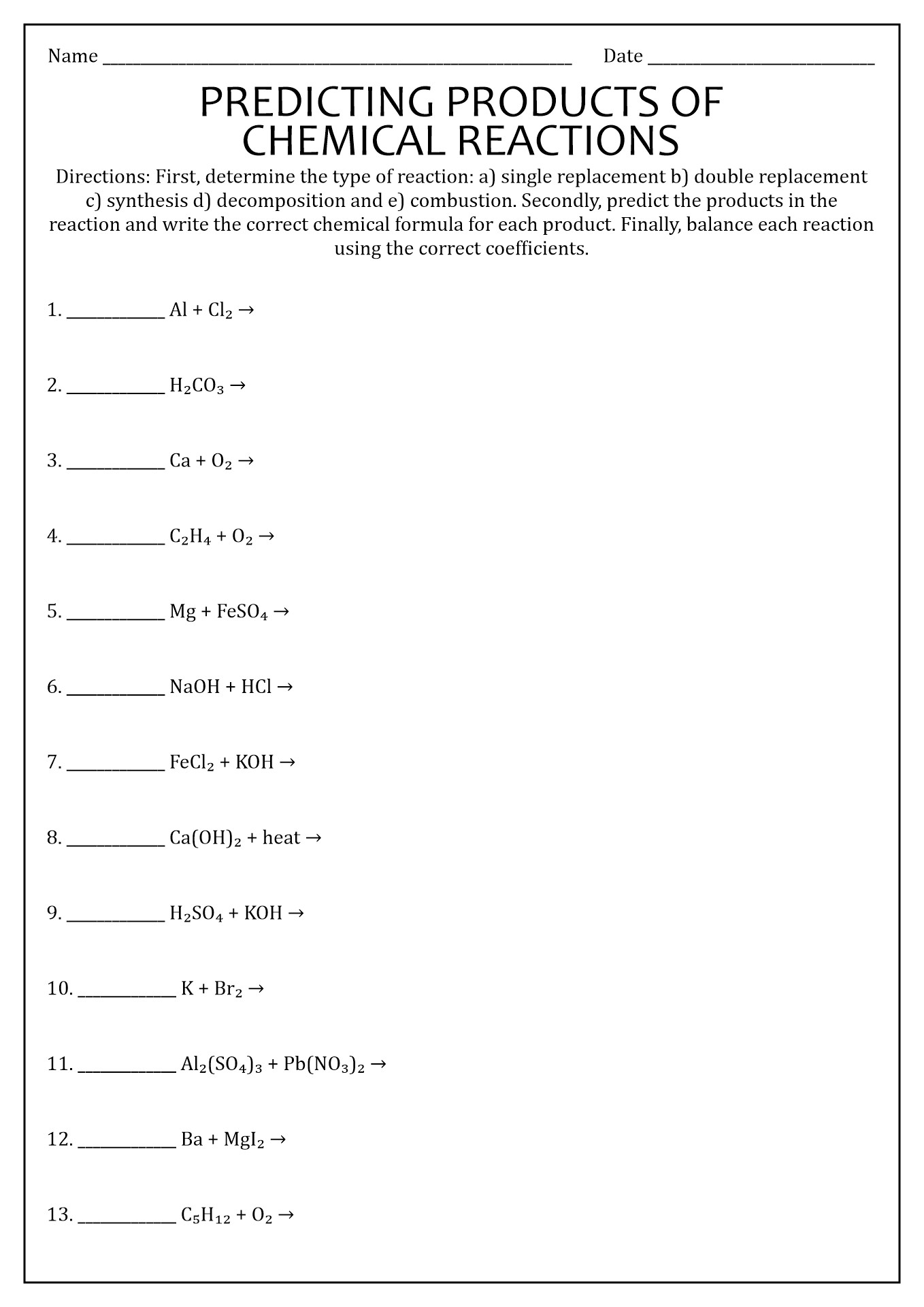
On a SEPARATE SHEET OF PAPER, write the reactants, predict the products, balance it, and then write the type of reaction. 1. Magnesium Bromide reacts with Chlorine à ??? MgBr2 + Cl2 à MgCl2 + Br2 balanced and single replacement reaction. 2. Aluminum reacts with Iron III Oxide à ??? 2 Al + Fe2O3 à Al2O3 + 2 Fe single replacement reaction. 3. 4.
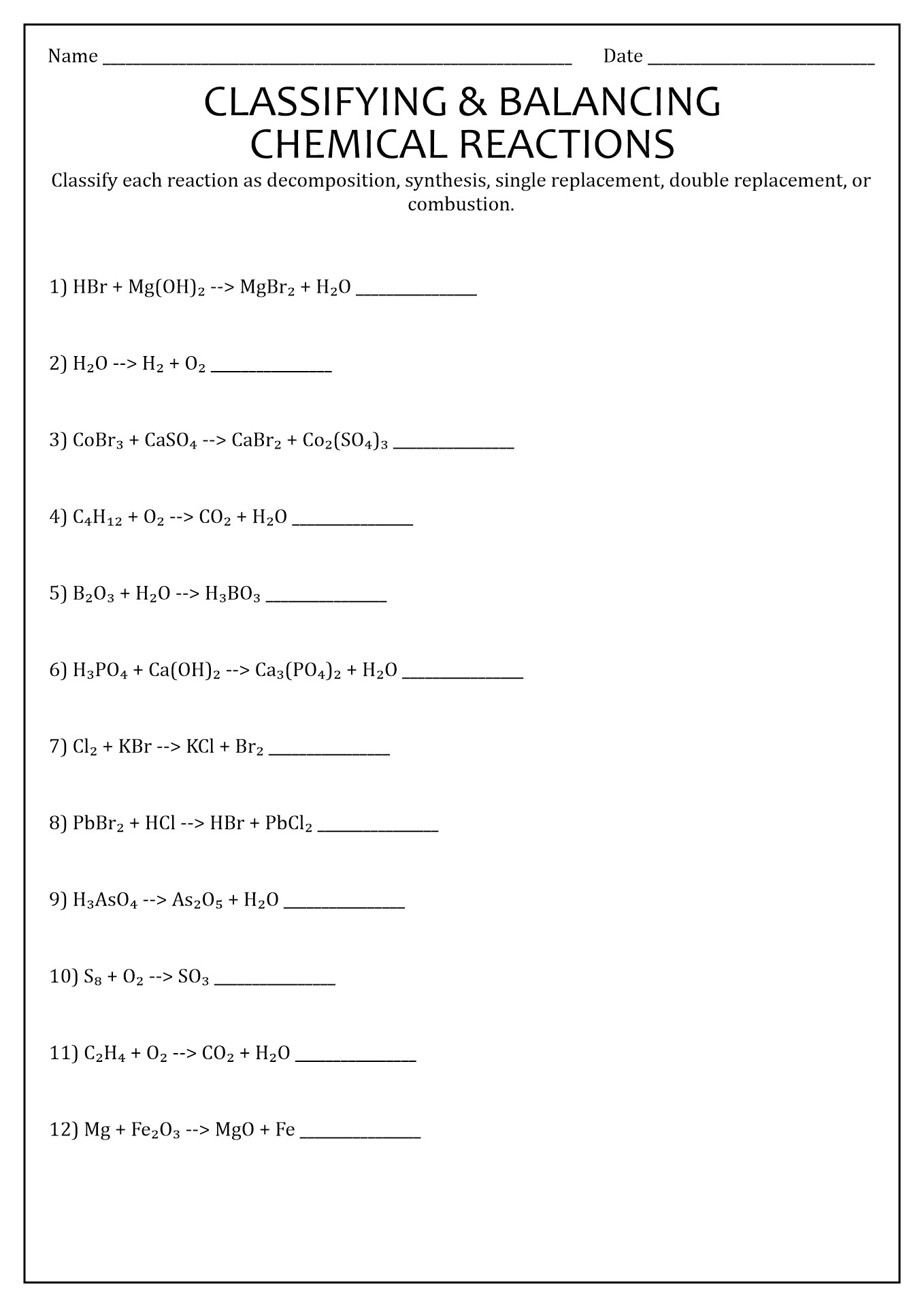
16 Types Chemical Reactions Worksheets Answers Free PDF at
predicting products of chemical reactions - practice problems. 13. Lithium metal reacts with liquid bromine. 14. Potassium metal reacts with silver chloride. 15. Sodium metal reacts with hydrochloric acid, HCl, and produces hydrogen gas as one of the products. 16. Solutions of tin (II) nitrate and potassium hydroxide are combined.
- Sp Authentic 22 23 Release Date
- Things To Do In Galveston Tx In January
- Never Let Go Of My Ex Husband
- Cottages In Brackley Beach Pei
- Mot De 6 Lettres Contenant T
- Quick Brown Fox Jumps Over The Lazy Dog Typing Test
- 3 Rowntree Road Condos For Sale
- Appartement A Louer St Hyacinthe 4 1 2
- Kia Seltos 2024 Bleu Pluton
- Cpl Flight Test Guide Pdf